
2024 Forecast for Metal Stamping Materials
The metal stamping industry is a critical component of the manufacturing sector, playing a vital role in producing parts for various industries, ...
Subscribe for updates to our blog
Implantable Medical Devices Spur Metal Stamping Innovations
The implantable medical device market is growing at a rapid pace globally. As manufacturers develop amazing new therapeutic technologies for ...

Continual Training Demonstrates a Commitment to Quality
This is the tenth and final post in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" ...
Corrective & Preventive Actions CAPA Reduce Med Device Quality Issues
This is the ninth in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

Improve Quality w/ Careful Supplier Selection, Monitoring & Evaluation
This is the eighth in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

Avoid Quality Issues by Implementing a Change Control Process
This is the seventh in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help ...

Prevention and Detection Systems Improve Quality Control
This is the sixth in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

Medical Device Firms Improve Quality w/ Launch/Qualification Process
This is the fifth in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

Costs of Quality Failure Makes Risk Management a High Priority
This is the fourth in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

In-House Eng, Design & Tool Build Improve Metal Stamping Reliability
This is the third in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

ISO 13485 Demonstrates Knowledge of Medical Device Specs and Reqt's.
This is the second in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

Top Management's Commitment to Quality Spreads Throughout Organization
This is the first in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

10 Metal Stamping Quality Indicators for Medical Device Mfrs
Quality is the #1 concern for OEM engineering and purchasing managers in all industries, but nowhere is quality more critical than in the ...

Sorting Out Quality Best Practices for Medical Metal Stampings
Highly specialized and regulated industries, such as the medical device industry, demand adherence to their own preferred standards for ...
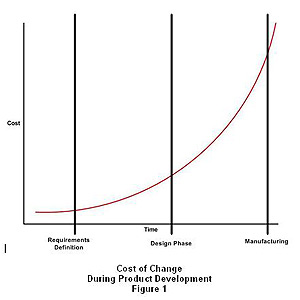
5 Tips to Smooth Transition from Medical Device Design to Manufacture
A common obstacle to a successful market launch is the transition of medical products from design to manufacture, with issues often leading to ...