
2024 Forecast for Metal Stamping Materials
The metal stamping industry is a critical component of the manufacturing sector, playing a vital role in producing parts for various industries, ...
Subscribe for updates to our blog

Metal Stamping Defects Can Spell Disaster for Medical Devices
Defective medical devices are costing manufacturers billions of dollars every year. Even more alarming, defects can lead to patient death and harm. ...

Ensuring Quality in Metal Stampings from Start to Finish: 13 Critical Steps to Ensure Metal Stamping Success (Steps 8-13)
Manufacturers who require precision metal stampings can benefit from understanding how to optimize the metal stamping process and reduce costs. This ...

Metal Stampings Can Impact Your Bottom Line: 13 Critical Steps to Ensure Metal Stamping Success (Steps 1-3)
Metal stamped parts may appear to be just a small component of the larger product, but they can have a significant impact on a manufacturer’s bottom ...

What to Look for at Site Visits to Metal Stamping Companies
Manufacturers who are considering adding or replacing a supplier of precision metal stamping face a daunting task of evaluation. Often, the process ...

Two Key Factors for Picking Quality Metal Stamping Suppliers
Every manufacturer’s goal is to have a zero-defect production line, but OEMs and their suppliers alike know that problems are inherent in any ...

Continual Training Demonstrates a Commitment to Quality
This is the tenth and final post in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" ...
Corrective & Preventive Actions CAPA Reduce Med Device Quality Issues
This is the ninth in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

Improve Quality w/ Careful Supplier Selection, Monitoring & Evaluation
This is the eighth in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

Avoid Quality Issues by Implementing a Change Control Process
This is the seventh in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help ...

Prevention and Detection Systems Improve Quality Control
This is the sixth in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

Medical Device Firms Improve Quality w/ Launch/Qualification Process
This is the fifth in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

Costs of Quality Failure Makes Risk Management a High Priority
This is the fourth in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

In-House Eng, Design & Tool Build Improve Metal Stamping Reliability
This is the third in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

ISO 13485 Demonstrates Knowledge of Medical Device Specs and Reqt's.
This is the second in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

Top Management's Commitment to Quality Spreads Throughout Organization
This is the first in Kenmode's blog article series entitled "10 Metal Stamping Quality Indicators for Medical Device Manufacturers" to help medical ...

10 Metal Stamping Quality Indicators for Medical Device Mfrs
Quality is the #1 concern for OEM engineering and purchasing managers in all industries, but nowhere is quality more critical than in the ...

Sorting Out Quality Best Practices for Medical Metal Stampings
Highly specialized and regulated industries, such as the medical device industry, demand adherence to their own preferred standards for ...

Does Your Precision Metal Stamper Practice MSA? Ask!
The Importance of Following MSA and GR&R Quality Standards It’s tempting to think that all precision metal stamping companies and component ...

Metal Stamping Companies Adopt Automotive Industry Best Practices
Quality Matters: Learning from the Turnaround in the U.S. Auto Industry For decades, the U.S. auto industry lagged behind foreign brands in ...

Auto Industry Averts Risk of Poor Metal Stamping Quality w/ APQP
Precision metal stampings are a critical component of so many products. Automotive components, medical devices, electronic parts – all contain metal ...
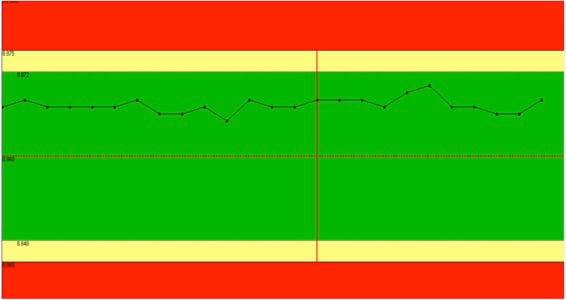
Want consistent precision metal stamping quality? Insist on SPC.
SPC. You’ve heard the acronym. Some customers, especially in the automotive industry, require it. But do you know why it’s critical for metal ...
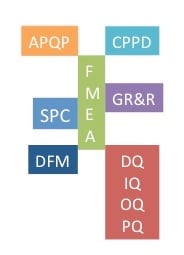
Precision Metal Stamping Quality -- Acronyms You've Got to Know.
As an engineer or designer, you expect precision metal stamping manufacturers to provide uncompromised quality. Zero defects is no longer a goal; it ...

A Must-Have Reference for Precision Metal Stamping Projects
Are you a design engineer, specifier, or buyer of precision metal stampings? Then you can't afford to be without this must-have reference.

WOW -- Metal Stamping Business Has Changed in 50 Years!
The precision metal stamping industry has changed just a bit in the last 50 years.